“r.itemList.length” “this.config.text.ariaShown”
” this.config.text.ariaClosed “
By David Bautz, PhD
OTC: APLIF TSX: APLI. Ⅴ
READ THE FULL APLIF RESEARCH REPORT
Business update
FDA approves expansion of favipiravir trial to save you COVID-19
On August 10, 2020, Appili Therapeutics Inc.(OTC: APLIF) (TSX: APLI.V) announced that the U.S. FDA would not be an issue in the U.S. But it’s not the first time You have the legal authority to expand your phase 2 clinical trial of favipiravir for the long-term prevention of COVID-19.long-term care facilities. Appili had already obtained regulatory approval from Health Canada in May 2020 to launch the test in Canada.
The ongoing coronavirus outbreak has affected older people the most, especially those living in long-term care facilities. In Canada, about 80% of all COVID-19 deaths occurred in long-term care centers, while in the United States, several states report that more than 50% of COVID-19 deaths occurred there.Therefore, finding a way to prevent the spread of COVID-19 among this population can have a significant effect on epidemic mitigation.
The randomized, partially blind, placebo-led group trial aims to recruit approximately 760 participants to 16 long-term care services, 8 services that administer favipiravir to their citizens, and the remaining 8 who administer placebo.two or more citizens have tested positive for SARS-CoV-2.After the installation is recruited, all citizens will get favipiravir or a placebo, the main result of the trial being that of the epidemic, explained how there are no new cases of COVID- 19 in citizens for 24 consecutive days until the 40th day after the onset of prophylaxis.Treatment.Secondary goals come with safety, infection rates, disease progression and mortality rates.The company recently announced that the on-site assessment of long-term care services has begun in Ontario.as soon as an outbreak of COVID-19 is confirmed.
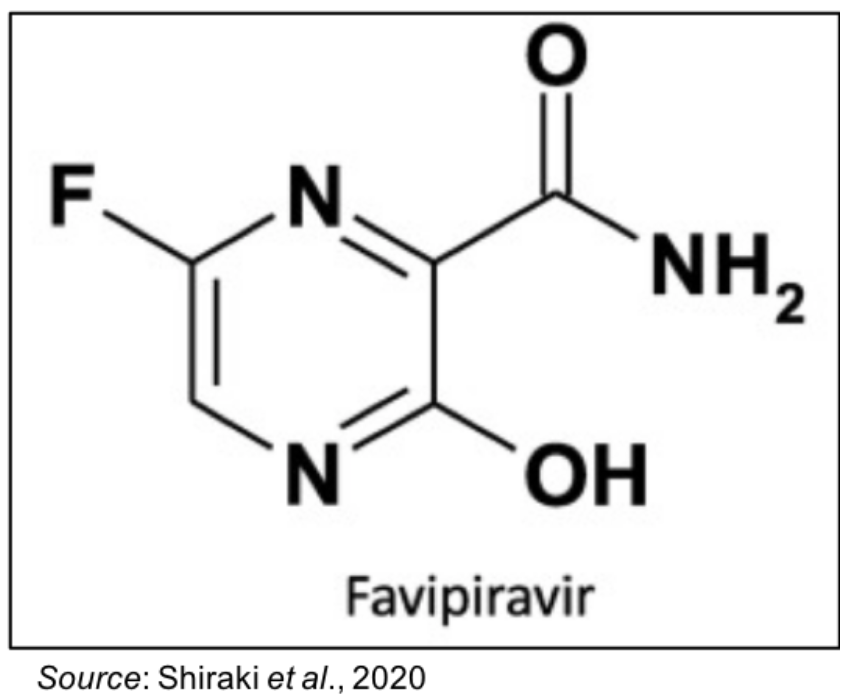
Favipiravir
favipiravir is a broad-spectrum antiviral compound that selectively inhibits influenza RNA-dependent RNA polymeria (RdRP) and many other RNA viruses (Shiraki et al., 2020). It was discovered through Toyoma Chemical Co., Ltd., which analyzed a giant chemical library for compounds with anti-influenza activity.Favipiravir has activity opposite influenza strains A, B and C, which include seasonal and pandemic strains (Fruruta et al., 2002).
The drug interferes with viral replication by joining RdRP and acting as a chain terminator at the onboarding site (Jin et al., 2013); however, compared to other antiviral compounds, it does not appear to generate resistant strains, for example, strains of the oseltamivir-resistant influenza virus (Tamiflu®) have given the impression, however, a favipiravir-resistant virus has never given the impression in phase 3 clinical trials (Takashita et al., 2016). This is encouraging, as the use of favipiravir will probably not lead to effective relief if the drug were used in the existing coronavirus pandemic.
The favipiravir has recently been approved for the pandemic influenza remedy in Japan under the logo called Avigan®.However, it is contracted in pregnant women as it has shown teratogenic and embryotoxic effects in animals.Other potential side effects come with nausea, vomiting, diarrhea and liver damage..
Favipiravir and COVID-19
There are a number of study teams around the global reading of favipiravir for the COVID-19 remedy, as demonstrated through the 14 ongoing or planned clinical trials to begin, indexed in Clinicaltrials.gov.So far, there have been two reports in the media trials in China and Japan that tested favipiravir in patients with COVID-19, however, it is worth noting that these studies analyzed the remedy with favipiravir.after patients had already been diagnosed with COVID-19.
Cai et al., 2020: This non-randomized open trial was conducted in Shenzhen, China and compared favipiravir with lopinavir / ritonavir (control arm) for coVID-19 remedy.The effects showed that patients treated with favipiravir had a viral elimination time than the control arm (4 days versus 11 days; Q
Chen et al., 2020: This randomized open trial was conducted in Wuhan, China and compared favipiravir with umifenovir (control arm) for the COVID-19 remedy.The number one endpoint was day 7 of clinical recovery, which did not differ particularly between the organization of favipiravir (71/116, 61%) and the control organization (62/120, 52%) (p – 0.1396); however, the remedy with favipiravir led to a shorter duration of fever (difference of 1.7 days; Q
These reports are encouraging and show that favipiravir has antiviral activity opposite SARS-CoV-2 with a mild side effects profile.
One-year primate knowledge is expected soon for ATI-1701
In early 2020, Appili presented positive interim knowledge for ATI-1701 at the ASM Biothreats 2020 convention organized through the American Society of Microbiology.The company had in the past presented similar effects at the convention on science and generation of chemical and biological defense in November 2019.Results presented in November 2019 showed that ATI-1701 rats (annotated clpB) for 365 days and cynomolgus macaques for 90 days after vaccination, as illustrated in the following figures.This was tested by comparing vaccinated animals with F.tularesnsis spray, resulting in a mortality of one hundred percent in unvaccinated rats, rats immunized with the F vaccine.(LVS) and unvaccinated macaques.
In addition to appearing that ATI-1701 provided a survival credit for immune animals, the following figure shows that immune animals had strong immune responses as shown through particularly superior production of interferon-gamma (IFN-g), fractalquine (CX3CL1), tumour necrosis thing (TNF) and interleukin-2 (IL-2) for inexperienced animals.
The findings were that ATI-1701 can be manufactured in a solid form and that it protects rats and macaques for at least 365 and 90 days, respectively.We expect the company to publish the 365-day coverage verification knowledge for macaques later.This year.
ATI-2307 Update
In November 2019, Appili acquired ATI-2307 from FUJIFILM Toyama Chemical Co., LTD.It is a new antifungal agent of broad-spectrum acryamidine that belongs to the same elegance of fragrant diamyinals as pentamidine and furamidine (Mitsuyama et al., 2008).It has a new highly differentiated mechanism of action that could potentially be used to treat infections caused by a number of clinical and high-priority pathogens, adding Cryptococcus, Candida and Aspergillus.
ATI-2307 has been effectively tested in multiple Phase 1 clinical trials, adding a Phase 1 clinical trial in healthy volunteers in Japan that showed that the drug was tolerated well (NCT02289599).Appili is obtaining evidence from non-clinical studies of concept to compare the healing effect of ATI-2307 in models of rabbit and mouse Cryptococcus intracranial infection along with comparing in vitro drug activity against a panel of clinical isolates.We anticipate that knowledge of these studies will be reported in 2020.
Financial update
On August 12, 2020, Appili announced its monetary effects for the first quarter of fiscal year 2021, which ended on June 30, 2020.Net loss for the first quarter of fiscal year 2021 CAD $2.6 million, or CAD $0.05 consistent with the stake, compared to a net loss of C$1.8 million, or C$0.06 consistent with the share, for the first quarter of fiscal year 2020.
R expenses
As of June 30, 2020, Appili had approximately C$25.6 million in money, money equivalents and short-term investments.In June 2020, the Company completed a public offering of approximately 12.9 million games worth C$1.20 consistent with the revenue unit of approximately Can$15.5 million.Each unit consisted of a non-unusual order consistent with percentage and an unusual portion consistent with percentage guarantee.Each order has a compensation value of C$1.50 and expires on June 10, 2023., 2020, the Company held a steady individual placement without a broker of 1.2 million sets to make gross profits of C$1.44 million.
As of June 30, 2020, Appili had approximately 61.6 million percentages in circulation, 15.3 million warrants and 4.0 million features for a fully diluted percentage count of approximately 81.0 million.
Conclusion
We look forward to updates on the phase 2 trial of favipiravir and believe it can have a significant effect on the coronavirus epidemic, as the elderly are obviously the most vulnerable and remedies to prevent the spread of the disease in this population are also looking for updates for the ATI-1701 and ATI-2307 systems over the next six months.In the absence of a change in style, our valuation remains at $2.50.
SIGN UP TO BUY SMALL CAP RESEARCH to get our articles and reports by email directly in the morning.Stop by our online page to learn more about Zacks SCR.
INFORMATION: Zacks SCR earned reimbursement from the issuer directly, an investment manager or an investor consulting firm, contracted through the issuer, for offering the study policy for a consistent period of at least one year.The study items, as seen here, are part of the service provided through Zacks and Zacks receives quarterly bills totaling up to $40,000 according to the year for those services.Full disclaimer HERE.