\n \n \n “. concat(self. i18n. t(‘search. voice. recognition_retry’), “\n
By M. Marín
NASDAQ: AEMD
READ THE FULL REPORT OF THE EMAD INVESTIGATION
Expand indications for hemopurification treatment
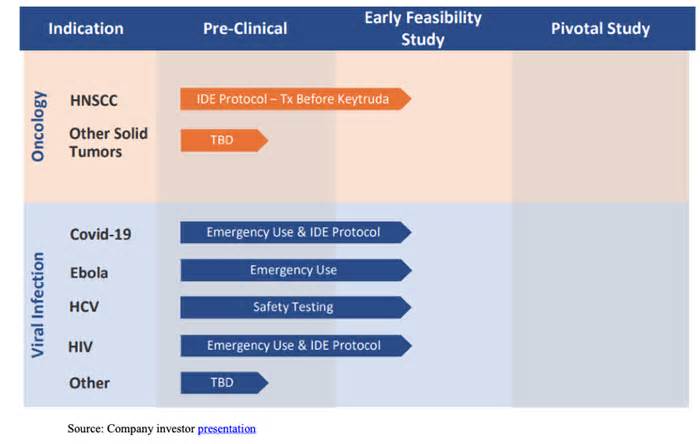
Aethlon Medical’s (NASDAQ:AEMD) clinical trials are progressing and expanding, as AEMD continues to demonstrate the efficacy of its leading product, Aethlon Hemopurifier®, in a wide variety of viruses and situations in single-patient emergency use and in vitro analysis, adding COVID-19 and variants and Monkey Pox, among others. Aethlon Hemopurifier® is being studied in a serious COVID-19 clinical trial under the company’s open Ide (research device exemption) for life-threatening viral infections.
The protection and viability of the hemopurifier is being evaluated in an early feasibility study (SAI) that will include up to 40 COVID-19 patients in intensive care. The first patient enrolled in this exam in June 2022 and finished hemopurifier treatment. The AEMD has nine fully activated hospitals that actively choose patients for the trial.
In addition to this study, Hemopurifier demonstrated positive effects in two critical patients in individual emergency use and in vitro analysis. Hemopurifier produced positive effects by joining seven variants of the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) virus in vitro, as reported in a paper1 contributed by Dr. Charles J. Fisher Jr. , CEO of AEMD, and Dr. Steven LaRosa, the company’s chief medical officer.
The company is also conducting a study on the effect of Hemopurifier on patients with head and neck cancer. The company is looking to expand this trial to one or more sites to increase patient enrollment. Publish a larger cancer trial to investigate the effect of Hemopurifier on a variety of cancerous tumors. Based on the effects of the trials, this could have broad economic and advertising implications for the MSA, in our view.
The device has been effective in treating monkeypox, with an increase
Recently, cases of monkeypox have increased. The WHO (World Health Organization) is closely monitoring MPV, as cases have been reported in several countries and the number of cases is increasing. In Nigeria, there has been an ongoing epidemic since 2017, according to the Harvard School of Public Health. In the second quarter of 2022, one death was reported in Nigeria. The Emergency Committee of the International Health Regulations met on 23 June 2022 in relation to the outbreak of monkeypox in several countries. Cases have been reported in about 50 countries, with the majority (more than 85%) reported in Europe and about 11% in North America. In addition, the EMD believes that possibly the actual number of cases would be underreported, as the disease and symptoms are not well known to doctors diagnosing at this stage.
Above all, Hemopurifier has proven its effectiveness against monkeypox. In 2008, the EMD commissioned the Battelle Memorial Institute to conduct an in vitro study of the ape pox virus (APV). The effects of this study demonstrated that peak MPV concentrations (approximately 35,000 cpu/ml) depleted the mobile culture fluids circulating in the Hemopurifier. Within 20 hours, the Hemopurifier had eliminated almost all (98%) of the infectious VPDs.
Specifically, knowledge indicated that Hemopurifier eliminated 44% of infectious VPDs within the first hour of testing, 82% after six hours, and 98% after 8 p. m. m. Studies were conducted in triplicate and knowledge verification was provided through a real-time polymerase chain reaction. .
Monkeypox study: elimination of infectious MPV via haemopurifier
▪ 44% in the first hour of testing
▪ 82% after six hours
▪ 9% after the afternoon
These in vitro and human treatment effects demonstrate the prospective versatility of Hemopurifier in the treatment of a variety of infections and tumors. They help the company’s development and clinical trial efforts around the device.
SUBSCRIBE TO ZENS SMALL CAP RESEARCH to receive our articles and reports directly by email every morning. Visit our online page to learn more about Zacks SCR.
DISCLOSURE: Zacks SCR obtained reimbursement directly from the issuer, an investment manager or an investor relations advisory firm, contracted through the issuer, for offering study policies for a consistent period of at least one year. Research articles, as stated herein, are part of the service provided through Zacks SCR and Zacks SCR receives quarterly invoices for a maximum payment amount of $40,000 consistent with the year for those provided to or in connection with the issuer. Full disclaimer HERE.
________________________
1. Elimination of clinically applicable SARS-CoV-2 variants by an affinity resin containing Galanthus nivalis agglutinin, in bioRxiv