n n n ‘. concat(e. i18n. t(“search. voice. recognition_retry”),’n
By M. Marín
NASDAQ:AMD
READ THE FULL AEMD RESEARCH REPORT
Pharmaceutical Authority of India Clearance Advances Goal of Studying Hemopurifier® in Patients With Solid Tumors
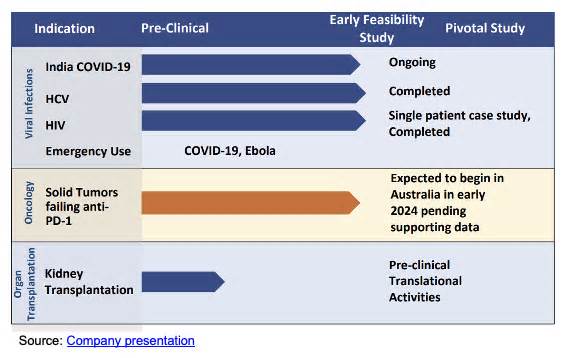
Aethlon Medical (NASDAQ: AEMD) has obtained approval from the Central Medicines Authority of India to conduct a Phase 1 trial of Hemopurifier® in patients treated with checkpoint inhibitor treatment. Despite the many advantages of checkpoint inhibitors, most patients do not respond to them. AEMD’s speculation is that the use of Hemopurifier in combination with checkpoint inhibitor treatment would possibly increase the percentage of patients who can benefit from it.
The Comptroller General of Medicines of India (DCGI), which is India’s central pharmaceutical authority, has authorized the company to conduct a Phase 1 trial on the safety, feasibility, and dose localization of Hemopurifier® in patients with sham tumors whose disease is solid or progressive. anti-PD-1 monotherapy remedy such as Keytruda® or Opdivo®. PD-1, or programmed cell death protein 1, is a protein discovered on the surface of cells that plays a role in inhibiting immune responses. A remedy like Keytruda®, a type of immunotherapy, helps block the PD-1 pathway to prevent cancer cells from hiding and thus helps the immune formula function. Although Keytruda® and other anti-PD-1 monotherapy treatments are a very important option for cancer treatment, they do not work on certain types of tumors in most cancer patients.
. . . to potentially help Hemopurify with checkpoint inhibitors possibly improve overall patient outcomes
For example, according to the NIH, “pembrolizumab (Keytruda® is the name of the logo) is a new first-line option for patients with complex NSCLC (non-small non-mobile lung cancer) and patients can achieve durable remission once they respond to it. But less than 30% of patients will respond to pembrolizumab, which means that most patients do not respond to checkpoint inhibitors. The company’s speculation is that the use of the Hemopurifier in conjunction with treatment with checkpoint inhibitors would possibly increase the percentage of patients who may respond to mixed treatment.
Prior to the test, AEMD will conduct an internal binding in vitro test of the applicable targets. The company can perform this test itself without having to hire an outside CRO or use outdoor lab facilities, which will keep costs down. In vitro studies have shown that Hemopurifier® captures exosomes that cause various types of cancer. This specific in vitro study will in particular compare Hemopurifier with PD-1 proteins. As noted, the company hopes that the Hemopurifier, in combination with an inhibitor, can help improve overall patient outcomes and demonstrate evidence of the concept of the device’s benefits in treating false tumors. The AEMD trial is expected to begin after the final touch of this in vitro study to verify that the applicable targets are held through the Hemopurifier.
The AEMD will then seek approval from the respective ethics committees of the interested sites in India. The company has a strong clinical relationship with Medanta Medicity Hospital in Delhi, India, which is lately participating in a hemopurifier study in COVID patients and we are not wondering if Medanta Medicity Hospital is interested in participating in the examination.
Checkpoint inhibitors like Keytruda have been used to treat more than 25 other types of cancer. With the launch of an oncology trial to investigate the effect of the Hemopurifier on various cancerous tumors, AEMD believes that it can generate knowledge that will help the hemopurifier improve outcomes, when combined with checkpoint inhibitor therapy, in several types of tumors where the cancer is related to extracellular cancer. Vesicles may advertise immune suppression and resistance to anti-PD-1 antibodies. Given the hemopurifier’s demonstrated ability to remove exosomes, the control believes the device can be used to improve outcomes in several types of cancer.
In addition, by focusing on multiple cancers, the company also expanded patient enrollment opportunities and created a database for regulatory approval. By adding Keytruda and Opdivo, we can also make patient selection easier.
. . . Planning a Similar Oncology Clinical Trial in Australia. . .
AEMD continues to work with the CRO (Contract Study Organization) overseeing its projects in Australia, NAMSA, to launch a similar oncology clinical trial in Australia; specifically, a safety, feasibility, and dose-finding trial in sham tumors in patients with anti-PD-1 antibody treatment failure. This is consistent with the company’s strategy of concentrating its clinical efforts first in Australia and India, where prices are particularly lower. The purpose of the company is to maximize its R&D expenses
Focus on the fields of oncology, organ transplantation, and infectious diseases.
. . . contributing to the increased availability of viable organs
The oncology study is also in line with Aethlon’s goal to focus on the oncology, organ transplant and then infectious disease spaces. The company recently announced an initiative to explore the hemopurifier’s ability to improve organ transplant outcomes, in addition to its existing studies on cancer and COVID-19. Aethlon will examine the hemopurifier’s ability to remove viruses and exosomes from harvested organs in order to increase the number of organs that can be effectively transplanted. The procedure can potentially decrease headaches such as viral infection. , delayed graft function, and even rejection after a transplant. Since the hemopurifier will be incorporated into the device’s existing perfusion organ preservation circuit, it will not increase the time for organ preservation, which is critical given that organs have a short window. that are still viable for transplantation.
Subscribe to ZACKS SMALL CAP RESEARCH to get our articles and reports emailed directly in the morning. Visit our online page to learn more about Zacks SCR.
DISCLOSURE: Zacks SCR obtained reimbursement from the issuer directly, from an investment manager, or from an investor relations advisory firm, contracted through the issuer, for providing policy studies for a consistent period of at least one year. Research articles, as noted here, are components of the service provided through Zacks SCR and Zacks SCR receives quarterly invoices totaling a maximum payment of up to $40,000 consistent with the year for what is provided to or with respect to the issuer. Full disclaimer HERE.